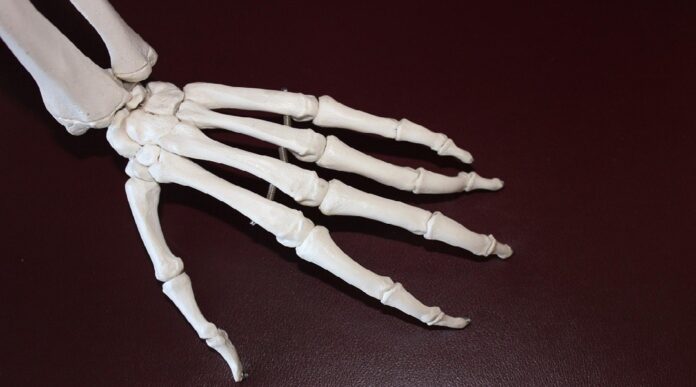
Scientists at the University of New South Wales have made significant progress in developing 3D-printed scaffolds that closely mimic the mechanical properties of natural bone. This breakthrough addresses a long-standing challenge in medicine: creating artificial bone replacements that perform as well as, or better than, existing metal implants or bone grafts. The new scaffolds exhibit superior strength, porosity, and fluid dynamics, bringing functional bone regeneration closer to reality.
The Problem with Current Bone Replacements
Traditional methods for repairing damaged bones often rely on metal implants or bone grafts. Metal implants, while strong, can be too rigid and stress surrounding tissues. Bone grafts, harvested from other parts of the patient’s body or from donors, are limited in availability and carry risks of rejection or infection. 3D printing offers a way around these limitations, but replicating the complex structure of bone has proven difficult.
Natural bone is both lightweight and strong, with a porous structure that supports cell growth and fluid flow. Early attempts at 3D-printed scaffolds often failed to balance these qualities, either collapsing under pressure or lacking the necessary porosity for tissue integration.
Mimicking Nature’s Design
Researchers overcame this hurdle by studying natural bone architecture. Real bone isn’t uniform; it transitions gradually from dense, load-bearing regions to lighter, sponge-like areas. The team replicated this graded structure using polylactic acid (PLA), a biocompatible and biodegradable plastic.
“We used a design approach inspired by natural bone. In bone, the structure changes gradually from dense areas to more open areas. We recreated this idea by printing scaffolds with graded structures in different directions.” – Dr. Juan Pablo Escobedo-Diaz
The resulting scaffolds, with approximately 55% porosity, showed remarkable performance in mechanical tests. They were 60% stronger and 16% stiffer under sudden impacts compared to slow, steady pressure, making them potentially ideal for load-bearing implants. The direction of the graded structure also influenced fracture patterns, giving designers another way to fine-tune material properties.
Fluid Flow and Future Implications
Importantly, fluids flowed through the scaffolds in ways that closely resemble natural bone, which is critical for nutrient delivery and waste removal during healing. While these scaffolds don’t yet possess the self-healing or adaptive capacity of living bone, they represent a major step forward.
Researchers anticipate clinical use within 5 to 10 years, pending further testing and regulatory approvals. Initially, they could be used in research and patient-specific modeling, eventually leading to replacements for large bone defects in areas like the femur. The team is now focusing on biomimetic designs that more closely replicate natural bone structures, including complex patterns and gradings.
This research highlights the potential of 3D printing to revolutionize bone regeneration, offering a future where artificial implants can not only support but also integrate with the body’s natural healing processes.